

报 告 人: Daesung Lee
报告时间: 2025-07-16上午10:00~上午11:30
报告地点: 嘉锡楼413
组织单位: 有机合成与功能福建省高校重点实验室
报告人简介:Daesung Lee,伊利诺伊大学芝加哥分校教授。Lee教授于1998年博士毕业于斯坦福大学,师从Paul A. Wender教授。1998-2000年在美国哈佛大学从事博士后研究(合作导师:Stuart L. Schreiber教授),2000年加入威斯康辛麦迪逊大学任助理教授,2007年加入伊利诺伊大学芝加哥分校任副教授,2014年任伊利诺伊大学芝加哥分校教授。2018-2021年,任温州大学兼职教授。迄今为止,Lee教授已在Nature, JACS, ACIE等国际著名期刊上发表研究论文150多篇。Lee教授获得Fellow of the A. P. Sloan Foundation等奖项。
报告摘要:
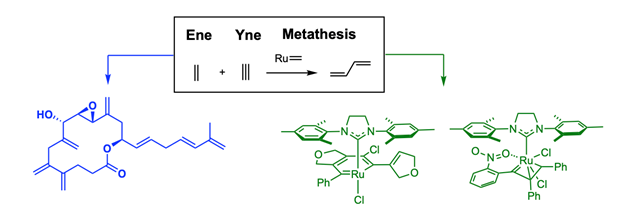
Enyne metathesis is a process by which alkene and alkyne functionalities are merged by the catalytic action of metal alkylidene complexes to form 1,3-diene as the product. We have developed various ring-closing or cross-enyne metatheses to construct molecules with extended unsaturation and apply them for the synthesis of various natural products, such as cochleamycin A, amphidinolide V. Ruthenium alkylidenes are known to undergo a so-called metallotropic [1,3]-shift if they are conjugated with an alkyne. Thus, the ring-closing or cross enyne metatheses can be streamlined with metallotropic [1,3]-shift if the reacting alkyne counterparts are conjugated with an additional alkyne moiety. Due to their effectiveness in forming various conjugated unsaturated systems such as 1,3-enynes, ene-1,3-diynes, ene-1,3,5-triynes, the enyne metathesis-induced metallotropic [1,3]-shift has been applied to the synthesis of epoxyquinoid natural products, including asperpetyne, harveynone, tricholomenyn A, and panaxytriol.
From an observation of the unusual stability of alkyne-conjugated alkylidene species, the chelation behaviors of alkene or alkyne-chelated ruthenium complexes have been examined. The steric and electronic impact of the substituents directly on the carbenic carbon or its periphery is found to be crucial to stabilize the alkene- or alkyne-chelated alkylidenes. Unexpectedly, disubstituted alkyne-chelated alkylidene complexes undergo facile cycloaromatization to form ruthenabenzenes, maintaining either a tetrahedral or square planar geometry depending on the ligand environment. These ruthenabenzene complexes show reliable metathesis reactivity, although they are slow in initiation, showing a latent behavior, so that in most cases the overall yields of the reactions are comparable to those with Grubbs complexes if enough reaction times and temperatures are permitted. In the presentation, the development of enyne metathesis and its application to natural product synthesis will be discussed, and how the new discoveries from the synthetic effort evolve into the research related to new ruthenium complexes, including ruthenabenzenes and catalytically viable ruthenium vinyl complexes, similar congeners of which were reported to be catalytically inactive.
References
1. Kim, M.; Lee, D. J. Am. Chem. Soc. 2005, 127, 18024.
2. Cho, E. J.; Lee, D. Org. Lett. 2008, 10, 257.
3. Voichkov I.; Lee, D. J. Am. Chem. Soc. 2013, 135, 5324.
4. Gupta, S.; Sabbasani, V.; Su, S.; Wink. D. J.; Lee, D. ACS Catal. 2021, 11, 1977.
5. Gupta, S.; Su, S.; Zhang, Y.; Liu, P. Wink. D. J.; Lee, D. J. Am. Chem. Soc. 2021, 143, 7490.